Study the following table in which positions of six elements A, B, C, D, E and F are shown as they are in the modem periodic table:
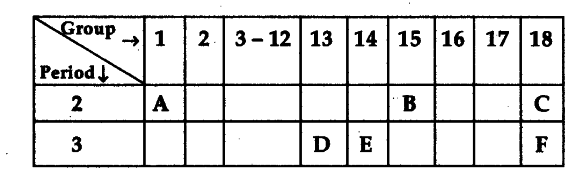
On the basis of the above table, answer the following questions:
(i) Name the element which forms only covalent compounds.
(ii) Name the element which is a metal with valency three.
(iii) Name the element which is a non-metal with ’ valency three.
(iv) Out of D and E, which is bigger in size and why ?
(v) Write the common name for the family to which the elements C and F belong.
(i) E
(ii) D
(iii) B
(iv) D is bigger; atomic radius/size decreases from left to right along a period.
(v) Noble gases.