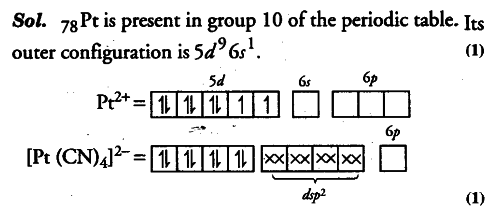
For square planar shape, the hybridisation is d{{sp}^{2}} . Hence, the unpaired electrons in 5d-orbital pair up to make one d-orbital empty for d{{sp}^{2}} hybridisation. Thus, there is no unpaired electron.

For square planar shape, the hybridisation is d{{sp}^{2}} . Hence, the unpaired electrons in 5d-orbital pair up to make one d-orbital empty for d{{sp}^{2}} hybridisation. Thus, there is no unpaired electron.