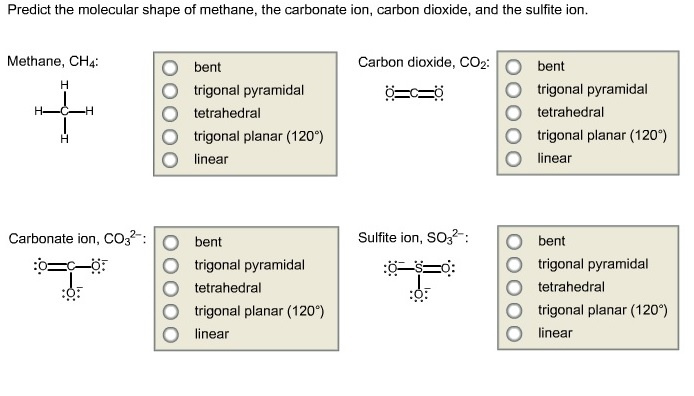
Predict the molecular shape of methane, the carbonate ion, carbon dioxide, and the sulfite ion.
Concepts and reason
This problem is based on the concept of chemical bonding.
VBT and VSEPR deal only with sigma bonds and valence electrons. There is no requirement of pi-bond in the process of determining the molecular shape.
Fundamentals
Theories such as VBT (valence bond theory) and VSEPR (valence shell electron pair repulsion theory) help in determining the shape, geometry and hybridization of a particular compound on the basis of various factors like valence bond, valence electrons and lone pairs.
Answer:
1)The molecular shape of the methane is given below:
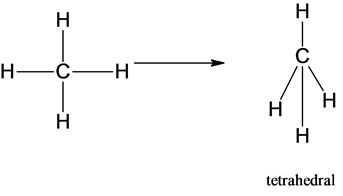
The molecular shape of the methane molecule is tetrahedral.
2)The molecular shape of carbonate is given below:
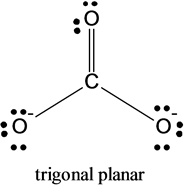
The molecular shape of the carbonate molecule is trigonal planar.
3)The molecular shape of carbon di oxide is given below:
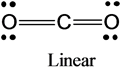
The molecular shape of the carbon di oxide molecule is linear.
4)The molecular shape of sulfite is given below:
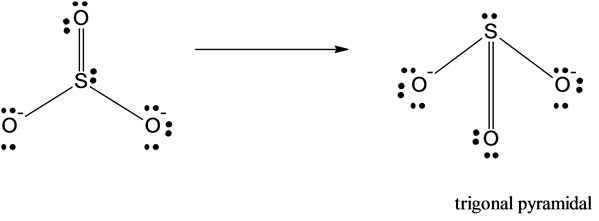
The molecular shape of the sulfite ion molecule is trigonal pyramidal.