Name the processes from which chlorine is obtained as a byproduct. What will happen if an aqueous solution of NaCl is subjected to electrolysis?
Down’s process is the process which gives chlorine as the byproduct.
Down’s process for the manufacture of Na metal When molten NaCl is subjected to electrolysis, chlorine is obtained as a byproduct at anode because in molten state, only {{Na}^{+}} and {{Cl}^{-}} ions are present.
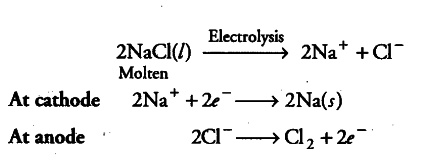
(ii) When aqueous solution of NaCl is electrolysed, {{H}^{+}} ions are reduced in preference to Na+at cathode and H _{ 2 } gas is released. {{Na}^{+}} remains in the solution and form NaOH with {{OH}^{-}} ions. Chlorine gas is obtained at anode as a byproduct.
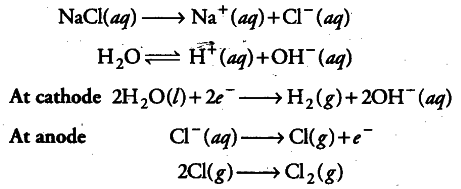
H _{ 2 } gas is obtained at cathode, chlorine gas at anode (as byproduct) and NaOH is formed in the solution.
![]()