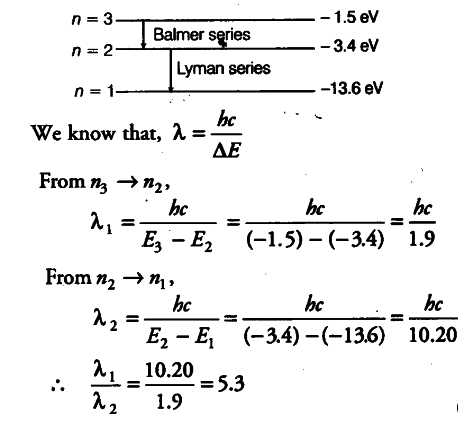
(i) In H-atom, an electron undergoes transition from second excited state to the first excited state and then to the ground state. Identify the spectral series to which these transitions belong.
(ii) Find out the ratio of the wavelengths of the emitted radiations in the two cases.
(i) An electron undergoes transition from second excited state to the first excited state which corresponds to Balmer series and then to the ground state which corresponds to Lyman series.
(ii) The wavelength of the emitted radiations in the two cases.