Look at figure and answer the following
questions:
(a) What change would you observe in the calcium hydroxide solution taken in tube B ?
(b) Write the reaction involved in test tubes A and B respectively.
© If ethanol is given instead of ethanoic acid, would you expect the same change ?
(d) How can a solution of lime water be prepared in the laboratory ?
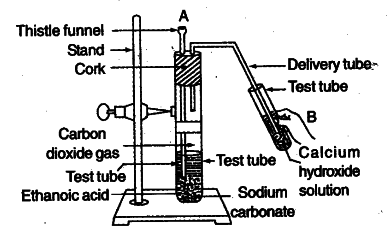
(a) It will turn milky.
(b) 2C$H_{3}$COOH + $Na_{2}$C$O_{3}$ ------->2C$H_{3}$COONa + $H_{2}$O+ C$O_{2}$ (Test tube A)
Ca($OH)_ {2}$+ C$O_{2}$ --------> CaC$O_{3}$ + $H_{2}$O
(Test tube B)
With excess C$O_{2}$, milkiness disappears. CaC$O_{3}$ + $H_{2}$O + C$O_{2}$ ---->Ca(HC$O_{3}$)_ {2}$
© As $C_{2}$$H_{5}$OH and $Na_{2}$C$O_{3}$ do not react, a similar change is not expected.
$C_{2}$$H_{5}$OH + $Na_{2}$C$O_{3}$ ------> No change
(d) The lime water is prepared by dissolving calcium oxide in water and decanting the supematent liquid.
