The value of $\Delta $fG° for the formation of $Cr_{2}O_{3}$ is - 540 kJ ${{mol}^{-1}}$ and that of $Al_{2}O_{3}$ is - 827 kJ ${{mol}^{-1}}$. Is the reduction of $Cr_{2}O_{3}$ possible with Al?
The two thermochemical equations may be written as:
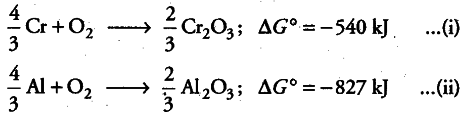
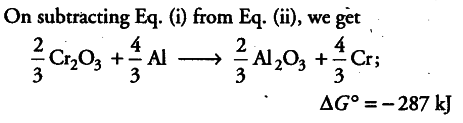
As $\Delta $fG° comes out to be negative, the reaction is feasible, i.e. Al can be used for the reduction of Cr_{2}O_{3}.