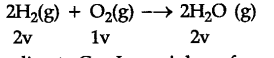
According to Gay Lussac’s law of gaseous volumes, 2 volume of dihydrogen react with 1 volume of {{O}_{2}} to produce 2 volume of water vapour. Therefore, 10 volume of dihydrogen on reaction with 5 volume of dioxygen will produce 10 volume of water vapour.