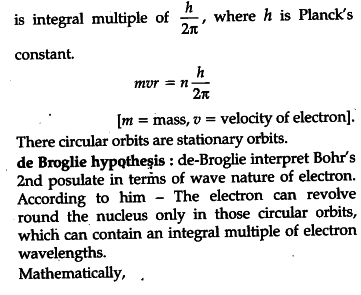
(i) State Bohr’s quantization condition for defining stationary orbits. How does de Broglie hypothesis explain the stationary orbits ?
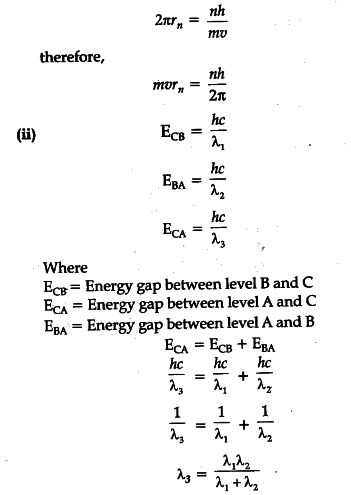
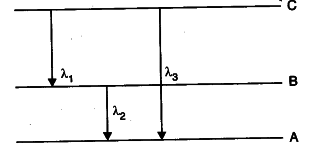
(ii) Find the relation between the three wavelength
{ \lambda }_{ 1 }
{ \lambda }_{ 2}
and { \lambda }_{ 3 } from the energy level diagram shown below.
(i) Bohr’s quantization condition: The electron can revolve round the nucleus only in those circular Orbits in which angular momentum of an electron