- When bonds are formed, energy is released and the system becomes more stable. If carbon forms four bonds rather than two, still more energy is released and so the resulting molecule becomes even more stable.
- The energy difference between the 2s and 2p orbitals is very small. When carbon atom, is ready to form bonds it gets a small amount of energy from bond energies and gets excited to promote an electron from the 2s to the empty 2p to give four unpaired electrons.
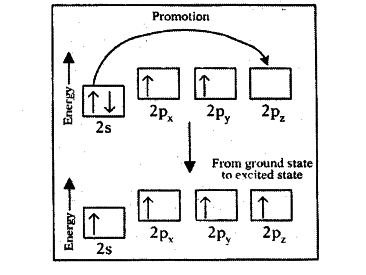
We have got four unpaired electrons ready for bonding, but these electrons are in two different kinds of orbitals and their energies are different.
4) We are not going to get four identical bonds unless these unpaired electrons are in four identical orbitals.