- When a conducting wire is connected to the terminals of the battery, a potential difference is created between the ends of the conductor.
- This potential difference sets up an electrical field throughout the conductor. The conductor contains large number of electrons.
- The electrons near the positive terminal of battery are attracted by it and start to move towards positive terminal.
- As a result, the amount of positive charge on the plate decreases.
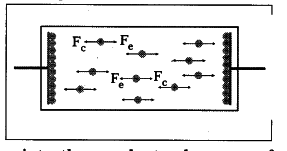
- So, the electric force F _{ e } becomes weaker
than chemical force F _{c} and the chemical force pulls negative ions from the positive plate (anode) and makes them move towards the negative plate (cathode). - The negative terminal pushes one electron into the conductor because of stronger repulsion between negative terminal and negative ion.
- Hence, the total number of electrons in the conductor remains constant during the current flow.
- The above process continues till equilibrium is attained between electric force [F _{ e }] and chemical force [F _{c}].