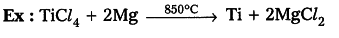Extraction of metals in the middle of the activity series :
The ores of these metals are generally present as sulphides or carbonates. Therefore prior to reduction of ores of these metals, they must be converted into metal oxides.
The metal oxides are then reduced to the corresponding metals by using the following methods :
- Reduction of metal oxides with carbon : The oxides are reduced by coke in closed furnace which gives the metal and carbon monoxide (CO).
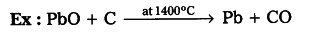
- Reduction of oxide ores with CO :

- Auto reduction of sulphide ores : In the extraction of copper from its sulphide ore, the ore is subjected to partial roasting in air to give its oxide.
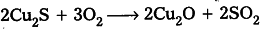
When the supply of air is stopped and increase temperature results in the reaction of rest of sulphide ore with oxide to form metal
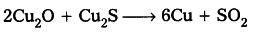
- Reduction of ores by more reactive metals : When highly reactive metals such as sodium, calcium, aluminium etc., are used as reducing agents, they displace metals of low reactivity from the compound.