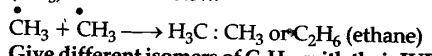One of the termination step during the formation of chlorination of methane involves the side reaction of the combination of two methyl free radicals (C${{H}_{3}}$) as shown below.
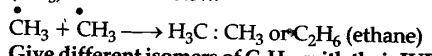
One of the termination step during the formation of chlorination of methane involves the side reaction of the combination of two methyl free radicals (C${{H}_{3}}$) as shown below.