The main points of difference between inductive and resonance effects are given below.
Inductive Effect :
- It involves displacement of only 0- electrons and hence occurs only in saturted compounds.
- During inductive effect the electron pair is only slighty displaced towards the more electronegative atom and hence only partial positive and negative charges appear.
- Inductive effects are transmitted over short distance in saturated carbon chains and the magnude of the effect decreses rapidly as the distance from the heteroatom increases. The effect almost becomes negligible beyond three carbon atoms from the heteroatom.
![]()
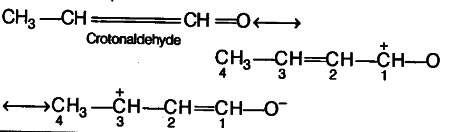
Resonance Effect :
- It involves delocalisation of jt or n (lone pairs) of electrons and hence occures in unsaturated and conjugated systems.
- During resonance effect, the electron pair is completely transferred and hence full positive and negative charge appear.
- The resonance effect are transmitted all along the length of the conjugated system without suffering much change in magnitude.