Give reasons for the following.
(i) Covalent bonds are directional bond while ionic bonds are non-directional.
(ii) Water molecule has bent structure whereas carbon dioxide molecule is linear.
(iii) Ethyne molecule is linear.
(i) A covalent bond is formed by the overlapping of atomic orbitals. The direction of overlapping gives the direction of bond. In ionic bond, the electrostatic field of an ion is non-directional. Each positive ion is surrounded by a number of anions in any direction depending upon its size and vice-versa.
That’s why covalent bonds are directional bonds while ionic bonds are non-directional.
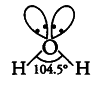
(ii) In H _{ 2 }O, oxygen atom is {{sp}^{3}} hybridised with two lone pairs. The four {{sp}^{3}} hybridised orbitals acquire a tetrahedral geometry with two corners occupied by hydrogen atoms while other two by the lone pairs.
The bond angle is reduced to 104.5° from 109.5° due to greater repulsive forces between lp — lp and the molecule thus acquires a V-shape or bent structure (angular structure).
In { CO }_{ 2 } molecule, carbon atom is sp-hybridised. The two sp hybrid orbitals are oriented in opposite directions forming an angle of 180°.
![]()
That’s why { H }_{ 2 }O molecule has bent structure whereas
{ CO }_{ 2 } molecule is linear.
(iii) In ethyne molecule, both the carbon atoms are sp hybridised having two unhybridised orbitals, i. e.,
2 px and 2 py. The two sp hybrid orbitals of both the carbon atoms are oriented in opposite direction forming an angle of 180°.
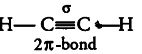
That’s why ethyne molecule is linear.