$FeSO_{4}$ solution mixed with solution in 1:1 molar ratio gives the test of
${{Fe}^{2+}}$ ion but $CuSO_{4}$ solution mixed with aqueous ammonia in 1 : 4 molar ratio does not give the test of ${{Cu}^{2+}}$ ion. Why?
When FeSO_{4} solution are mixed in 1:1 molar ratio, Mohr’s salt (a double salt) is formed.
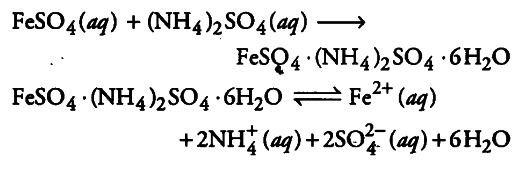
Because {{Fe}^{2+}} ions are formed on dissolution of Mohr’s salt, its aqueous solution gives the test of {{Fe}^{2+}} ions,But when CuSO_{4} is’ mixed with ammonia, following reaction occurs.