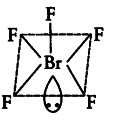
The central atom Br has seven electrons in the valence shell. Five of these will form bonds with five fluorine atoms and the remaining two electrons are present as one lone pair. Hence, total pairs of electrons are six (5 bond pairs and 1 lone pair). To minimize repulsion between lone pairs and bond