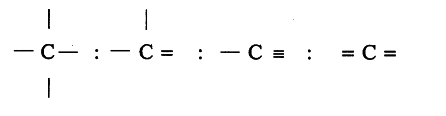Electronic configuration of carbon (ground state) :
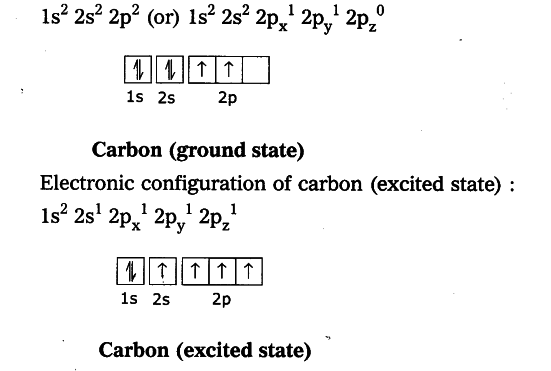
In excited state of carbon atom one ‘2s’ electron is promoted to ‘2p _{ z }’ orbital. Each carbon in excited state has four unpaired electrons and forms four covalent bonds as shown below.
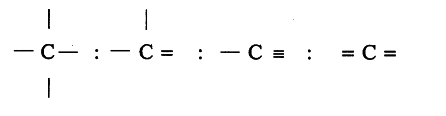
Electronic configuration of carbon (ground state) :

In excited state of carbon atom one ‘2s’ electron is promoted to ‘2p _{ z }’ orbital. Each carbon in excited state has four unpaired electrons and forms four covalent bonds as shown below.