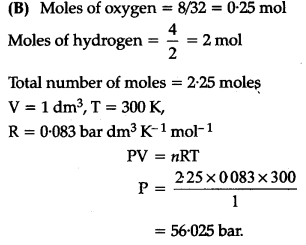Explain the following :
Boyle’s Law
(ii) Avogadro’s Law
(iii) Critical temperature.
(B) Calculate the totalpressure in a mixture of 8 g of oxygen and 4 g of hydrogen confined in a vessel ( of 1 d${{m}{3}}$ at 27°C. R = 0.083 bar d${{m}{3}}$ ${{K}^{-1}}$ ${{mol}^{-1}}$.
(A) (i) Boyle’s law : According to Boyles law, at a constant temperature, the volume of a given mass of a gas is inversely proportional to its pressure, i.e.,if, volume increases the pressure would decrease. This is because as volume increases, the number of molecules striking the walls of a container in a given time decreases leading to decrease in pressure. (ii) Avogadro’s Law: It states that equal volumes of all gases under the same conditions of temperature and pressure contains equal number of molecules.
(iii) Critical temperature : A gas may be liquified by decreasing the temperature and increasing the pressure. The temperature above which a gas cannot be liquify how so ever high the pressure may be is called critical temperature.