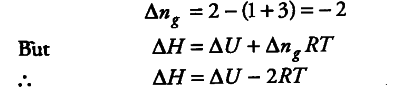Establish a relationship between ∆H and ∆U in Haber’s process of synthesis of ammonia assuming that gaseous reactants and products are ideal.
Haber’s process of synthesis for ammonia is
{ N }_{ 2 }+
3 { H }_{ 2 } ----------->
2${ NH }_{ 3 }$
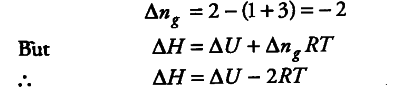
Establish a relationship between ∆H and ∆U in Haber’s process of synthesis of ammonia assuming that gaseous reactants and products are ideal.
Haber’s process of synthesis for ammonia is
{ N }_{ 2 }+
3 { H }_{ 2 } ----------->
2${ NH }_{ 3 }$