![]()
The structures of cis- and trans- isomers of hex-2-ene are
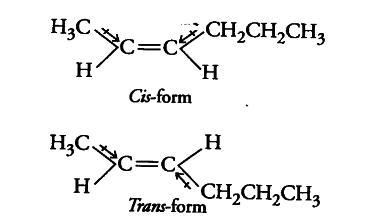
Cis- form is more polar than trans-form i.e., cis-form has higher dipole moment than trans-form. Thus, the boiling point of cis-isomer is greater than that of trans-isomer because of the greater dipole-dipole interactions between the molecules in it. further more trans-isomer of hex-2-ene is almost non-polar.