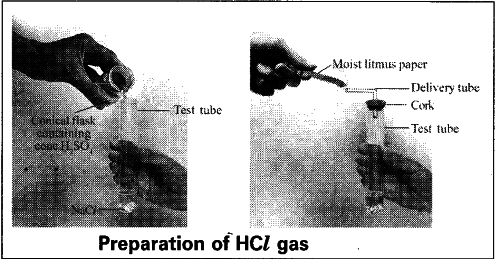
- Take about 1.0 g of solid NaCl in a clean and dry test tube.
- Add some concentrated sulphuric acid to the test tube.
Observation : - A gas is coming out of the delivery tube.
- If we test the gas with dry and wet blue litmus paper there is no change in colour.
Chemical equation :

Conclusion : - We can conclude that dry HCl gas (hydrogen chloride) is not an acid.
- Because we have noticed that there is no change in colour of dry litmus paper.
- But HCl aqueous solution is an acid because wet blue litmus paper turned into red.