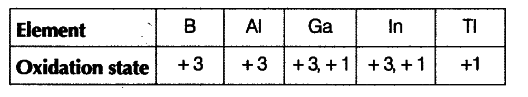
Boron and aluminium show an oxidation state of +3 only because they do not exhibit inert pair effect due to the absence of d or /-electrons. Elements from Ga to Tl show two oxidation states, i.e.,+ Land +3. The tendency to show +1 oxidation state increases down the group due to the inability of {{ns}^{2}} electrons of valence shell to participate in bonding which is called inert pair effect.
Therefore, {{Tl}^{+}} is more stable than {{Tl}^{3+}} .