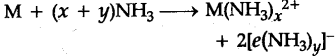General characteristics of the alkaline earth ; metals :
Group 2 of the periodic table comprises the elements beryllium, magnesium, calcium, strontium, barium and radium. These (except beryllium) are known as the alkaline earth metals,
(i) Atomic Properties of Alkaline Earth Metals.
(a) Electronic configuration: Electronic configuration of the metal M = (Noble gas) n {{s}^{2}};
(b) Atomic and ionic size : The atomic and ionic radii of these metals are smaller than those of alkali metals and from top to bottom, the atomic and ionic sizes increase.
© Ionisation Enthalplies : The first ionisation enthalpies of the alkaline earth metals are higher than those of alkali metals, because of increase in nuclear charge.
From top to bottom, ionisation energies decrease with the addition of one orbit each time.
(ii) Physical Properties of Alkaline Earth Metals.
(a) Physical appearance : These metals are generally silvery white, lustrous and relatively soft, but harder than the alkali metals. Be and Mg are somewhat greyish.
(b) Melting and boiling point: The fairly higher melting and boiling points of the alkaline earth metal compared to those of the corresponding alkali metal are attributed of their smaller sizes and two valence electrons.
© Flame colorations : Chlorides of alkaline earth metal (except that of Be and Mg) produce characteristics colour to the flame due to easy excitation of electron to higher energy levels.
(iii) Chemical Properties of Alkaline Earth Metals.
(a) The alkaline earth metals are less reactive than the corresponding alkali metals. The reactivity of these elements increases on going down the group.
(b) Reactivity towards the halogens: All the alkaline earth metals react with halogens at elevated temperature forming halides
![]()
© Reactivity towards hydrogen : Except Be, all other alkaline earth metals combine with {{H}_{2}} upon heating to form hydrides.
![]()
(d) Reactivity towards acids : The alkaline earth metal readily react with acids liberating {{H}_{2}} gas.
![]()
(e) Reducing nature : Like alkali metals, the alkaline earth metals are strong reducing agents, but their reducing power is less than that of alkali metals. Generally reducing character increases from top to bottom.
(f) Solutions in liquid ammonia : Like alkali metals, the alkaline earth metals dissolve in liquid ammonia to give deep blue black solutions