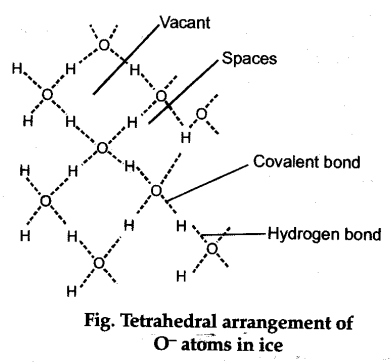
At atmospheric pressure, ice crystallises in the normal hexagonal form. In it each oxygen atom is tetrahedrally surrounded by four oxygen atoms. This gives rise to an open cage like structure with strong covalent bonds (shown by solid lines) and Hydrogen bonds.