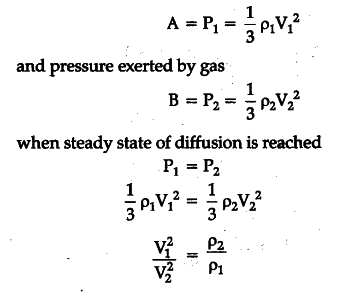
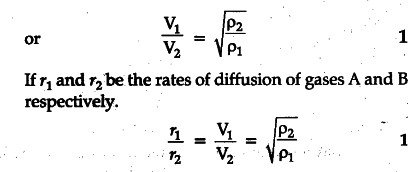It states that the rate of diffusion of a gas is inversely proportional to the square root of its density. 1 Let us consider two gases A and B diffusing into one another. Let P1 and P2 be their densities and V1 and V2 their respective r.m.s. velocities. Pressure exerted by gas