Compounds such as alcohols and glucose contain hydrogen but are not cat-egorized as acids. Describe an activity to prove it.
Aims : To prove that solutions of alcohols and glucose are not acids eventhough they contain hydrogen.
Procedure :
- Prepare solutions of glucose, alcohol, hydrochloric acid and sulphuric acid.
- Drill two holes on a rubber cork and introduce two iron nails into the holes.
- Connect two different coloured electrical wires and keep it in a 100 ml beaker.
- Connect free ends of the wire to 6V battery and complete the circuit.
- Pour some diluted HCl in the beaker and switch on the current.
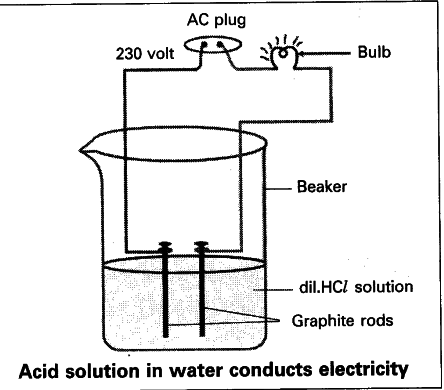
- We find that the bulb glows.
- Repeat the activity with diluted H_{2}SO_{4}, glucose and alcohol solutions separately.
- The bulb glows when acid is taken in the beaker. But the bulb does not glow when glucose and alcohol are taken in the beaker.
- Glowing of bulb indicates that there is flow of electric current through solution.
- Acid solutions have ions and the movement of these ions helps flow of current
but glucose and alcohol solutions do not have free ions and so current does not pass through them.