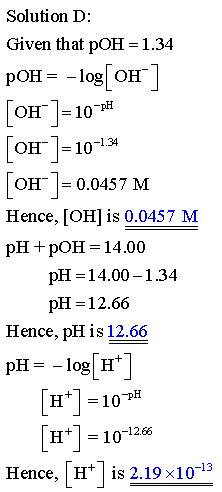
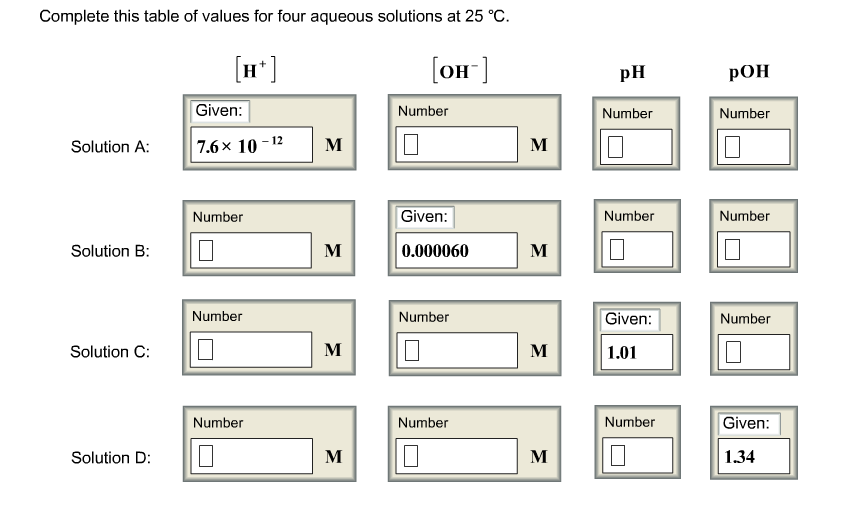
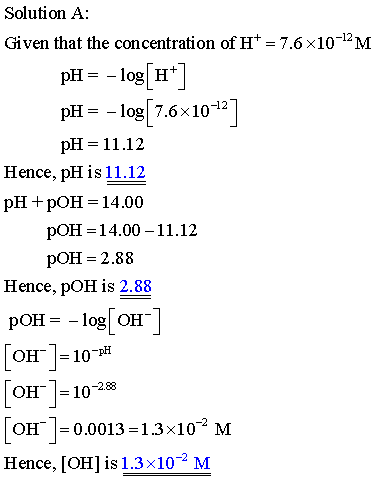Complete this table of values for four aqueous solutions at 25 C.
Answer:
PH of the solution is the negative logarithm of the hyrogen ion concentration.
![]()
POH of the solution is the negative logarithm of the hydroxide ion concentration
![]()
The relation between PH and POH is as follows: