Comment on the thermodynamic Stability of NO(g), given:
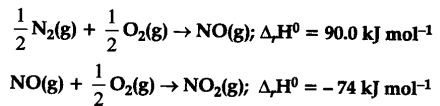
As can be seen from the first reaction, formation of NO is endothermic process and will take only when 90 K ${{mol}^{-1}}$ energy is available. However, the second reaction involving the oxidation of NO to N${{O}{2}}$ is an exothermic process and energetically more feasible. Therefore NO(g) is unstable and it would get converted into N${{O}{2}}$ (g) spontaneously.