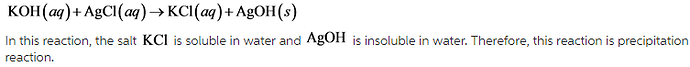Classify each of these reactions.
- Ba(ClO3)2(s)—>BaCl2(s)+3O2(g)
- 2NaCl(aq)+K2S(aq)—>Na2S(aq)+2KCl(aq)
- CaO(s)+CO2(g)—>CaCO3(s)
- KOH(aq)+AgCl(aq)---->KCl(aq)+AgOH(s)
- Ba(OH)2(aq)+2HNO2(aq)—>Ba(NO2)2(aq)+2H2O(l)
each classify reaction should be either one of this.
acid�base neutralization
precipitation
redox
none of the above
Concepts and reason
The concept used to solve this problem is based on chemical reaction.
In a chemical reaction, two or more substances combine to form a new substance by forming or breaking a bond.
Fundamentals
Acid and base react to form salt and water this is known as acid-base neutralization reaction. Redox reaction is a chemical reaction which involves the transfer of electrons between two species. It is also called oxidation-reduction reaction.
Precipitation reaction is a reaction in which anions and cations combine to form an insoluble ionic solid in aqueous solution.
Answer:
Part - 1
The reaction mention in the question is as follow.
![]()
In this reaction, chlorine is reduced and oxygen is oxidized. Both reduction and oxidation occur in this reaction. Therefore, this is a redox reaction.
Part 1
[Answer]
The reaction is redox reaction.
In this reaction, because chlorine loses the electrons so it is a reducing agent and oxygen gains electrons so it is an oxidizing agent. Therefore, it is a redox reaction.
Part - 2
The reaction mention in the question is as follow.
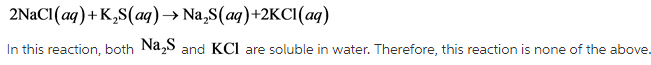
Part 2
[Answer]
The reaction is none of the above.
A double displacement reaction is a reaction in which two compounds react and cations and anions exchange their places to form new compounds.
Part - 3
The reaction mention in the question is as follow.
Part 3
[Answer]
The reaction is none of the above.
Part - 4
The reaction mention in the question is as follow.
Part 4
[Answer]
The reaction is precipitation reaction.
In this reaction, both reactants react to form insoluble ionic solid. Therefore, it is a precipitation reaction.
Part - 5
The reaction mention in the question is as follow.
![]()
In this reaction, nitrous acid acts as an acid and barium hydroxide acts as a base. So, barium hydroxide and nitrous acid react to form salt and water. Therefore, this reaction is acid-base reaction.
Part 5
[Answer]
The reaction is acid-base neutralization reaction.
Acid and base react to form salt and water this is known as acid-base neutralization reaction.