Calculate the emf of the cell in which the following reaction takes place.
Ni (s) + 2 ${{Ag}^{+}}$(0.002 M) --------> ${{Ni}^{2+}}$ (0.160 M) + 2Ag (s)
Given that E°cell = 1.05 V
From the given cell reaction and Nernst
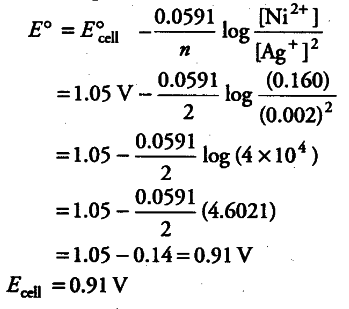
Calculate the emf of the cell in which the following reaction takes place.
Ni (s) + 2 ${{Ag}^{+}}$(0.002 M) --------> ${{Ni}^{2+}}$ (0.160 M) + 2Ag (s)
Given that E°cell = 1.05 V
From the given cell reaction and Nernst
