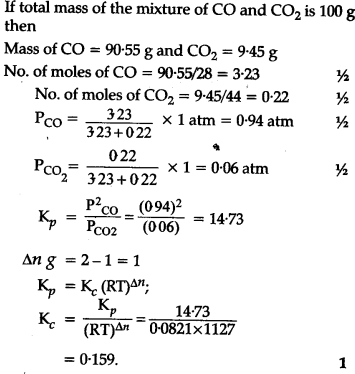
At 1127 K and 1 atm pressure, a gaseous mixture of CO and C${{O}{2}} in equilibrium with solid carbon has 90-55% CO by mass <img src="/uploads/db3785/original/2X/a/a6d1adcb54a2548f6a95b0eb8882680025a4b7db.png" width="202" height="23"> Calculate {{K}{c}}$ for this reaction at the above temperature ?