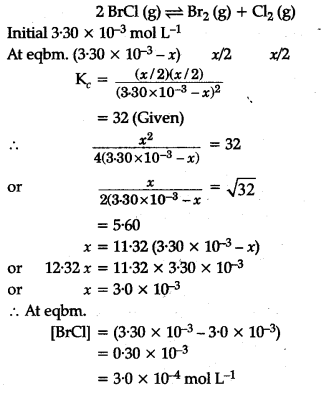
Bromine monochloride (BrCl) decomposes into bromine and chlorine and attains the equilibrium
![]()
for which {{K}_{c}} = 32 at 500 K.
If initially pure BrCl is present at a concentration of 3.30 x ${{10}^{-3}}$${{mol}^{-1}}$, what is its molar concentration in the mixture at equilibrium ?