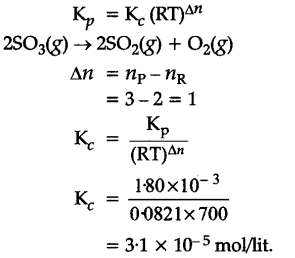At 700 K, the equilibrium constant {{K}_{p}} for the reaction
![]()
is (1-80 x {{10}^{-3}} K${{P}_{a}}$). What is the numerical value in moles per litre of IQ for this reaction at the same temperature ?
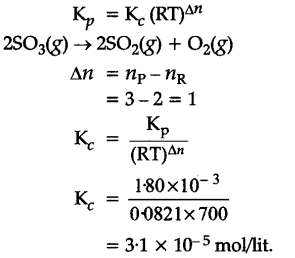
At 700 K, the equilibrium constant {{K}_{p}} for the reaction
![]()
is (1-80 x {{10}^{-3}} K${{P}_{a}}$). What is the numerical value in moles per litre of IQ for this reaction at the same temperature ?