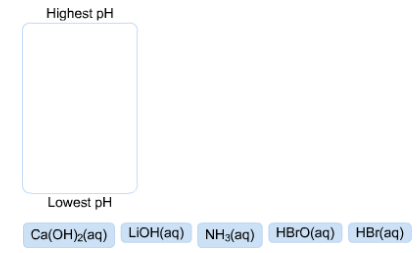
Assuming equal concentrations, rank these solutions by pH. Highest pH Lowest pH Ca(OH)2(aq) LiOH(aq) NH3(aq) HBrO(aq) HBr(aq)
Answer:
Concepts and reason
Write the dissociation reactions of the given acids and bases. Count the number of hydride and hydroxide ion given by each of the species.
Arrange the given species on the basis of number of hydride and hydroxide ions produced by the given bases and acids.
The species which are present in equilibrium are weak species.
Fundamentals
pH is defined as the negative logarithm of the hydrogen ion concentration. The scale of pH is from 0 to 14. The solutions in the pH range 0-6 are acidic in nature and the solutions in the pH range 8-14 are basic in nature. A solution with pH of 7 is said to be a neutral solution.
Strong acids and bases undergo complete dissociation in the solution. On dissociation of strong acids and bases,![]() and
and ![]() are produced in the solution which are responsible for the acidity and basicity of the solution.
are produced in the solution which are responsible for the acidity and basicity of the solution.
The solution which produces maximum ![]() is the most acidic solution.
is the most acidic solution.
The solution which produces maximum ![]() is the most basic solution.
is the most basic solution.
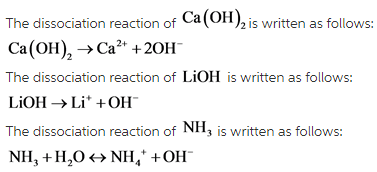

![]() dissociates to give 1 hydroxide ion.
dissociates to give 1 hydroxide ion.
![]() is a weak base. It undergoes partial dissociation in solution to give one hydroxide ion. The hydroxide ion does not increase the pH to a large extent because the dissociation of
is a weak base. It undergoes partial dissociation in solution to give one hydroxide ion. The hydroxide ion does not increase the pH to a large extent because the dissociation of ![]() is equilibrium controlled.
is equilibrium controlled.
