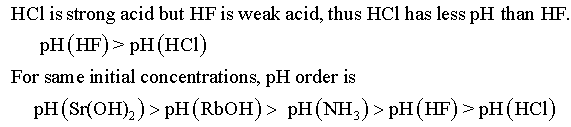
Assuming equal concentrations, rank these solutions by pH. Highest pH(1) Lowest pH(5)
RbOH(aq), Sr(OH)2(aq), HCl(aq), HF(aq), NH3(aq), from highest to lowest.
Answer:
Acids have pH value less than 7, bases have pH values more than 7.
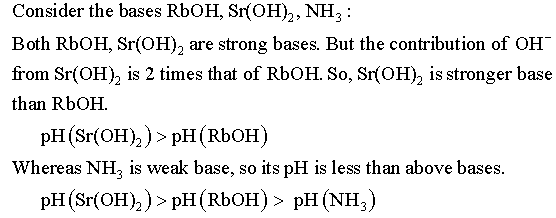
Consider the acids HF, HCI: