Assign oxidation number to the underlined elements in each of the following species
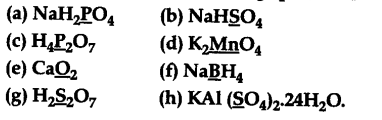
(a) Let the oxidation no. of P in Na${{H}{2}}$P${{O}{4}}$ be x.
- 1 + 2(+ 1) + x + 4(- 2) =0
- 1 + 2 + x-8 =0
x = + 5
Oxidation number of P in Na${{H}{2}}$P${{O}{4}}$ = + 5.
(b) Let the oxidation no. of S in NaHS${{O}_{4}}$ be x. - 1 + 1 + x + 4(- 2) =0
- 2 + x - 8=0
x = + 6
Oxidation number of S in NaHS${{O}{4}}$ = + 6.
© Let the oxidation number of P in ${{H}{4}}$${{P}{2}}$${{O}{7}}$ be x.
4(+ 1) + 2x + 7(-2) =0 - 4 + 2x -14 =0
2x =10
x = + 5
Oxidation number of P in ${{H}{4}}$${{P}{2}}$${{O}{7}}$ = + 5.
(d) Let the oxidation no. of Mn in ${{K}{2}}$Mn${{O}_{4}}$ be x.
2(+ 1) + x + 4(- 2) =0 - 2 + x-8 =0
x = + 6
Oxidation number of Mn in ${{K}{2}}$Mn${{O}{4}}$ = + 6.
(e) Let the oxidation number of oxygen in Ca${{O}_{2}}$ be x. - 2 + 2x =0
[oxidation no. of Ca = + 2]
2x = -2
x =-1
Oxidation number of O in Ca${{O}{2}}$ =-1.
(f) Let the oxidation no. of B in NaB${{H}{4}}$ be x. - l+x + 4(-l) =0
[•.• oxidation no. of H = -1]
x-3 =0
x = + 3
Oxidation number of B in NaB${{H}{4}}$ = + 3.
(g) Let the Oxidation no. of S in ${{H}{2}}$${{S}{2}}$${{O}{7}}$ be x.
2(+1)+ 2x + 7(-2) =0 - 2 + 2x -14 =0
2x =12
x = + 6
Oxidation number of S in ${{H}{2}}$${{S}{2}}$${{O}{7}}$ = + 6.
(h) Let the oxidation no. of S in KAl(S${{({{O}{4}})}{2}}$).24${{H}{2}}$O = x - 1 + 3 + 2x + 8(- 2) + 24(+ 2) + 24(- 2) = 0
- 4 + 2x -16 = 0
[O. No. of K = 1,O. No. of Al = 3]
2x =12
x = +6
Oxidation number of S in KAl(S${{({{O}{4}})}{2}}$).24${{H}_{2}}$O=+6