Arrange the following elements from greatest to least tendency to accept an electron.
Rank from greatest to least tendency to accept an electron. To rank items as equivalent, overlap them
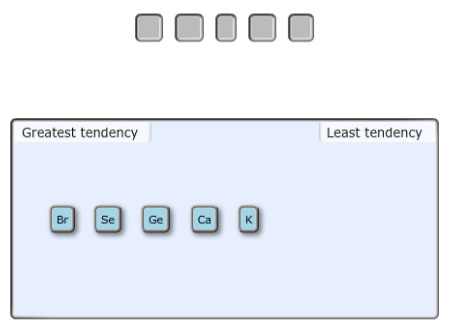
Answer:
The highest electron affinity is of Bromine atom (Br) that belongs to halogen family after that non-metal that is selenium (Se). Germanium (Ge) is metalloid and have less electron affinity than non-metals. Metals such as potassium (K) and calcium (Ca) will have least electron affinity.
In the outer most shell of Potassium and Calcium there is one or two electrons respectively.
They can easily loss these electrons to complete the octets therefore, they have minimum electron affinity but among two electron affinities of potassium is more as it has outer electronic configuration which is less stable than electronic configuration of calcium.
