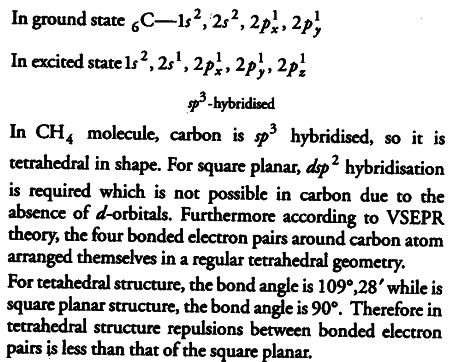
Apart from tetrahedral geometry, another possible geometry for ${ CH }{ 4 }$ is square planar with the four H-atoms at the corners of the square and the C atom at its centre. Explain, why
${ CH }{ 4 }$ is not square planar?
Electronic configuration of carbon