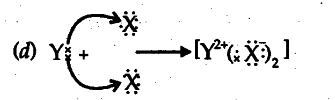An element X (atomic number 17) reacts with an element Y (atomic number 20) to form a divalent halide.
(a) Where in the periodic table are elements X and Y placed ?
(b) Classify X and Y as metal(s), non-metal(s) of metallpid(s).
© What will be the nature of oxide of element Y ? Identify the nature of bonding in the compound formed.
(d) Draw the electron dot structure of the divalent halide,
(a) X belongs to Group 17 and 3rd Period.
Y belongs to Group 2 and 4th Period.
(b) X—Non-metal and Y—Metal.
© Basic oxide; Ionic bonding