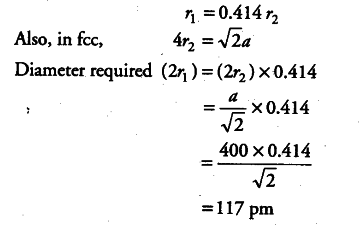An element crystallises in fee lattice having edge length 400 pm. Calculate the maximum diameter of atom which can be placed in interstitial site without distorting the structure.
In a cubic crystal system, there are two types of voids known as octahedral and tetrahedral voids. If rx is the radius of void and r2 is the radius of atom creating these voids then
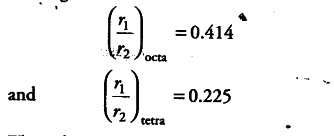
The above radius ratio values indicate that octahedral void has larger radius hence, for
maximum diameter of atom to be present in interstitial space.