Account for the following:
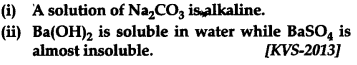
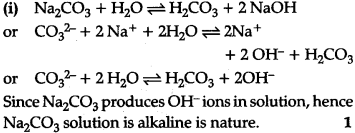
(ii)(ii) The lattice enthalpy of Ba${{SO}{4}} is much more than its hydration enthalpy and hence it is insoluble in water But hydration enthalpy of Ba{{OH}{2}} is much more than lattice enthalpy Therefore, Ba{{OH}_{2}}$ is soluble in water