Account for the following.
(i) $p{ k }_{ b }$ of aniline is more than that of methylamine.
(ii) Ethylamine is soluble in water whereas aniline
i) In aniline, the electron pair on nitrogen atom is involved in conjugation with ring and is less available for protonation than that in methylamine. Therefore, p{ k }_{ b } value of aniline is more than that of methylamine and aniline is less basic (as higher the pkb value, weaker is the base).
(ii) Ethylamine is soluble in water due to hydrogen bonding.
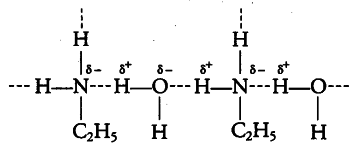
In aniline due to bulky hydrocarbon part, the extent of hydrogen bonding is less and it is not soluble in watet.