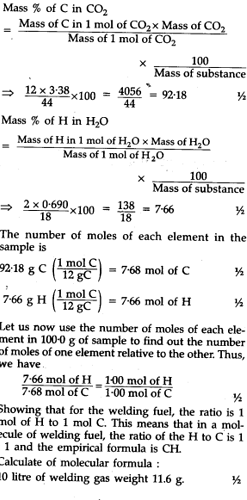
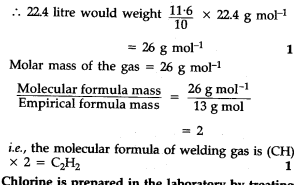
A welding fuel gas contains carbon and hydrogen only. Burning a small sample of it in oxygen gives 3.38 g carbon dioxide, 0.690 g of water and no other products. A volume of 10.0L (measured at STP) of this welding gas is found to weight 11.6 g. Calculate (i) empirical formula (ii) molar mass of the gas and (iii) molecular formula