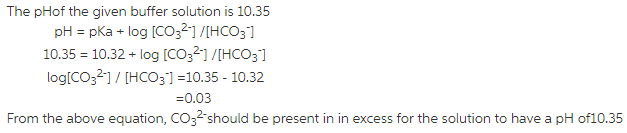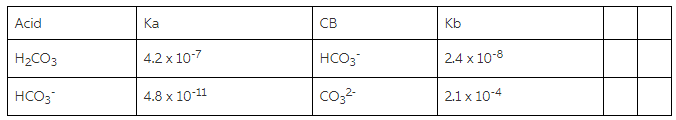
You are to prepare a “carbonate” buffer with pH of 10.35. Youare given carbonic acid (H2CO3). sodiumhydrogen carbonate (NaHCO3), and sodium carbonate(Na2CO3).
H2CO3 + H2O <----> H3O+ +HCO3-
HCO3- + H2O <—> H3o+ +CO3-
CO32- + H2O <—>HCO3- + OH-
a) Using the information in the chart about Ka/Kb values, whichspecies will you use? Explain your choice?
b) Which component will be present in the larger amount?
c) You are given a 0.025M solution of the acid and a 0.025Msolution of the conjugate base. Calculate how many mL of each youwould use to make the buffer.
Answer:
The pH of the buffer solution is given by the HendersonHasselbalch equation as:
pH = pKa + log [Base] / [Acid]
pH of the carbonate buffer is given to be 10.35 and thepH of the buffer solution lies close to the pKa value of the acidiccomponent of the buffer and hence the acidic componenet of thebuffer to be prepared should be ![]()