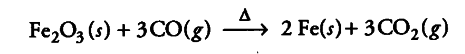A tetravalent element forms monoxide and dioxide with oxygen. When air is passed over heated element (1273 K), producer gas is obtained. Monoxide of the element is a powerful reducing agent and reduces ferric oxide to iron. Identify the element and write formulas of its monoxide and dioxide.
Write chemical equations for the formation of producer gas and reduction of ferric oxide with the monoxide.
Producer gas is a mixture of CO and { N }_{ 2 }, therefore, the tetravalent element is carbon and its monoxide and dioxide are CO and
{{CO}_{2}} respectively.
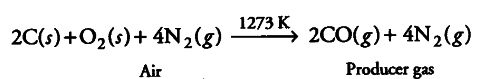
The carbon monoxide is a strong reducing agent and reduces ferric oxide to iron.