(a) (i) Name the Scientist who proposed this model of atom.
(ii) Write the three postulates of this model.
(iii) How many maximum electrons can be accommodated in M orbit ?
(b) What are canal rays ? Give the characteristics of canal rays.
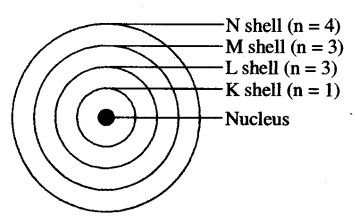
(a) (i) It is Neils Bohr’s Model of atom.
(ii) Postulates of Bohr’s Model:
- Positively charged nucleus is present in’ the centre of the atom.
- Electrons revolve around the nucleus in fixed circular paths called shells or orbits which are named as K, L, M, N,… or 1,2, 3,4.
- Shells are also called as energy levels as each shell is associated with a fixed amount of energy.
- Electrons do not loose or gain energy while revolving in a specific shell.
(iii) M shell can have 18 electrons.
(b) Canal rays are the positively charged radiations which consist of positively charged particles.
Or
The invisible positively charged radiations coming from anode are called canal rays. E. Goldstein discovered canal rays.
Characteristics of canal rays:
(i) They carry positively charged particles.
(ii) They travel in a straight line.
(iii) They have charge equal in magnitude but opposite in sign to that of electron.