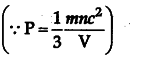On raising the temperature the average velocity of the gas molecule increases. As a result of which more molecules collide with the wall of the cylinder per second and hence greater momentum is transferred to the wall per second. Due to both these reasons the pressure increases.