2 moles of Zinc reacts with a cupric chloride solution containing 6.023 x 1022 formula units of Cu{C{l}_{2}} Calculate the moles of copper obtained
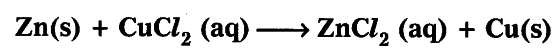
2 moles of Zinc reacts with a cupric chloride solution containing 6.023 x 1022 formula units of Cu{C{l}_{2}} Calculate the moles of copper obtained
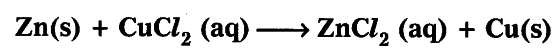
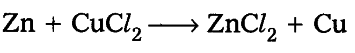
According to balanced equation 1 mole of Zinc requires 1 mole of cupric chloride to give 1 mole of copper. When two mole of zinc reacts with 1 mole of cupric chloride even then one mole of copper is obtained because copper chloride gets exhausted and excess zinc remains as it is.