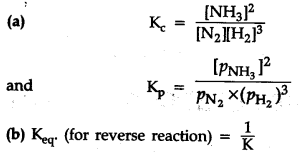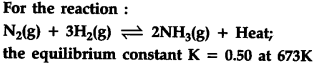
1.Write the expression for and {{K}_{p}}
2.What will be the equilibrium constant value for the reverse reaction at 673 K ?
3.What is the effect of increasing temperature on the yield of N${{H}{3}} ?
4.What is the effect of adding {{N}{2}}(g) and {{H}{2}}(g) on the yield of N{{H}{3}} ?
5.What is the effect of increasing pressure on the yield of NH3?
Or
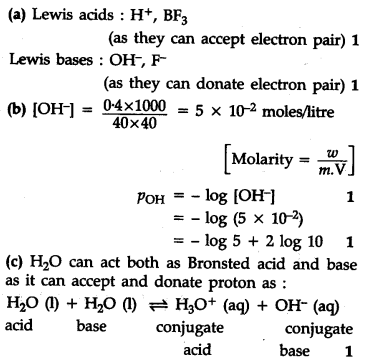
Classify the following species into Lewis acids and Lewis bases: O{{H}^{-}},{{F}^{-}},{{H}^{+}},B{{F}{3}} Calculate the pH of a solution in which 0.4 g of NaOH is dissolved in water to give 200 mL of solution.
6.{{H}{2}}$0 can act both as Bronsted arid and base. Write it’s corresponding conjugate base and conjugate arid.